The following chemical equation is not balanced. What must be done to the equation to make it balanced? 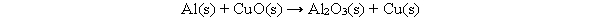
A) Change the coefficients to: 2 for Al, 3 for CuO and 3 for Cu.
B) Change the subscripts of CuO to CuO3.
C) Add a molecule of AlO.
D) Change the state of matter in the reactants.
E) Change the subscripts of Al2O3 to be AlO.
Correct Answer:
Verified
Q7: Combustion reactions are _ because products of
Q15: Under what circumstances is mass conserved?
A) Mass
Q33: In the body, glucose is broken down
Q36: How many atoms of magnesium are present
Q55: Which of the following reactions is balanced?
A)
Q56: The balanced equation for the combustion of
Q58: Your friend combines vinegar (which contains acetic
Q60: During metabolism, table sugar (sucrose) is broken
Q61: A common, over-the-counter antacid is Al(OH)3. This
Q64: The steps for performing stoichiometry calculations are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents