The steps for performing stoichiometry calculations are diagramed below: 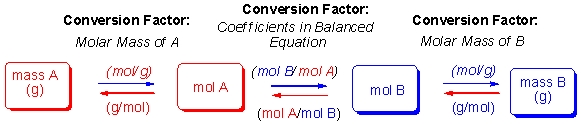 According to these steps, which of the following equations would allow you to calculate the yield of monosaccharides when 28 g of sucrose is broken down as shown in the following equation?
According to these steps, which of the following equations would allow you to calculate the yield of monosaccharides when 28 g of sucrose is broken down as shown in the following equation? 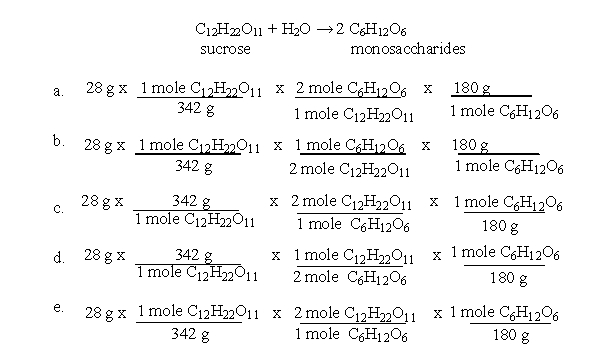
A) a
B) b
C) c
D) d
E) e
Correct Answer:
Verified
Q7: Combustion reactions are _ because products of
Q36: How many atoms of magnesium are present
Q39: How many atoms of hydrogen are present
Q56: The balanced chemical equation for the combustion
Q59: The following chemical equation is not balanced.
Q60: During metabolism, table sugar (sucrose) is broken
Q61: A common, over-the-counter antacid is Al(OH)3. This
Q65: Pentane (C5H12) reacts with oxygen gas (O2)
Q68: Pentane (C5H12) reacts with oxygen gas (O2)
Q69: The name for the heat energy released
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents