According to the balanced chemical equation below, if you react 3.0 mole of butene with 3.0 mole of hydrogen, how many moles of butane would you expect to get? 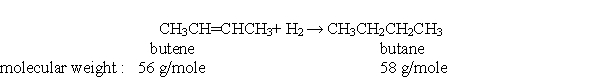
A) 0.0 moles
B) 1.0 mole
C) 2.0 moles
D) 3.0 moles
E) 6.0 moles
Correct Answer:
Verified
Q13: By moving, breathing, and living, you do
Q53: Is this chemical equation balanced? Mg(OH)2 +
Q69: The name for the heat energy released
Q70: According to the balanced chemical equation below,
Q72: Pentane (C5H12) reacts with oxygen gas (O2)
Q73: Acetylene (C2H2) is a small organic molecule
Q75: Which of the following biological molecules are
Q78: The balanced chemical equation for the combustion
Q79: According to the balanced chemical equation below,
Q88: How many atoms of oxygen are present
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents