When the following redox equation is balanced with smallest whole number coefficients, the coefficient for nitrogen dioxide will be _____. 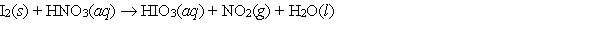
A) 1
B) 2
C) 4
D) 10
E) None of these choices is correct.
Correct Answer:
Verified
Q4: Consider the following balanced redox reaction
Q10: When the following redox equation is balanced
Q11: A voltaic cell is prepared using
Q12: A voltaic cell prepared using aluminum
Q13: Consider the following redox equation 
Q16: Which of the following statements about
Q17: Consider the reaction: CuO(s) + H2(g)
Q18: Which one of the following pairs of
Q19: When the following redox equation is balanced
Q38: Which component of the following cell notation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents