When the following redox equation is balanced with smallest whole number coefficients, the coefficient for zinc will be _____. 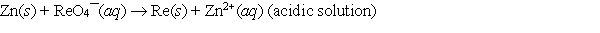
A) 2
B) 7
C) 8
D) 16
E) None of these choices is correct.
Correct Answer:
Verified
Q2: Which of the following statements about
Q4: Consider the following balanced redox reaction
Q5: A voltaic cell prepared using zinc
Q7: When the following redox equation is balanced
Q8: Which one of the following statements about
Q11: A voltaic cell is prepared using
Q12: A voltaic cell prepared using aluminum
Q13: Consider the following redox equation 
Q14: When the following redox equation is balanced
Q38: Which component of the following cell notation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents