A voltaic cell prepared using zinc and iodine has the following cell notation. 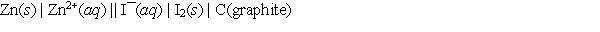 Which of the following equations correctly represents the balanced, spontaneous cell reaction?
Which of the following equations correctly represents the balanced, spontaneous cell reaction?
A) 2I¯(aq) + Zn2+(aq) I2(s) + Zn(s)
B) I2(s) + Zn(s) 2I¯(aq) + Zn2+(aq)
C) 2I¯(aq) + Zn(s) I2(s) + Zn2+(aq)
D) I2(s) + Zn2+(aq) 2I¯(aq) + Zn(s)
E) None of these choices, since graphite must be in the equation.
Correct Answer:
Verified
Q1: A voltaic cell prepared using aluminum
Q2: Which of the following statements about
Q3: Which one of the following is
Q5: Consider the following balanced redox reaction
Q7: When the following redox equation is balanced
Q8: Which one of the following statements about
Q10: When the following redox equation is balanced
Q11: A voltaic cell is prepared using
Q20: Which of the following solids is commonly
Q38: Which component of the following cell notation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents