You are given pure samples of pentane, , and 1,3-pentadiene, What prediction would you make concerning their standard molar entropies at 298 K?
A) S pentane > S 1,3-pentadiene
B) S pentane < S 1,3-pentadiene
C) S pentane S 1,3-pentadiene
D) S pentane S 1,3-pentadiene + 2*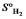
E) More information is needed to make reasonable predictions.
Correct Answer:
Verified
Q17: Which, if any, of the following
Q18: Which of the following is true
Q19: Which relationship or statement best describes
Q20: Which of the following values is
Q21: For a chemical reaction to be
Q23: Calculate
Q25: Which relationship or statement best describes
Q26: Calculate
Q26: Which one of the following changes of
Q40: In which one of the following pairs
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents