A mixture of 0.600 mol of bromine and 1.600 mol of iodine is placed into a rigid 1.000-L container at 350 C. 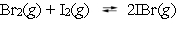 When the mixture has come to equilibrium, the concentration of iodine monobromide is 1.190 M. What is the equilibrium constant for this reaction at 350 C?
When the mixture has come to equilibrium, the concentration of iodine monobromide is 1.190 M. What is the equilibrium constant for this reaction at 350 C?
A) 3.55 * 10¯3
B) 1.24
C) 1.47
D) 282
E) 325
Correct Answer:
Verified
Q50: At high temperatures, carbon reacts with O2
Q51: The following reaction, in CCl4 solvent,
Q52: Ammonium iodide dissociates reversibly to ammonia
Q53: At 25
Q54: A mixture of 0.500 mole of carbon
Q56: The equilibrium constant Kc for the
Q57: At 25
Q58: Nitric oxide is formed in automobile
Q59: At a certain temperature the reaction CO2(g)
Q60: Compounds A, B, and C react
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents