The following reaction, in CCl4 solvent, has been studied at 25 C. 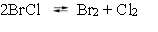
The equilibrium constant Kc is known to be 0.141. If the initial concentration of chlorine is 0.0300 M and of bromine monochloride is 0.0200 M, what is the equilibrium concentration of bromine?
A) 1.35 *10¯3 M
B) 2.70 * 10¯3 M
C) 8.82 * 10¯3 M
D) 9.70 * 10¯2 M
E) None of these choices is correct.
Correct Answer:
Verified
Q46: The reaction system Q48: 10.0 mL of a 0.100 mol L¯1 Q49: At 850 Q50: At high temperatures, carbon reacts with O2 Q52: Ammonium iodide dissociates reversibly to ammonia Q53: At 25 Q54: A mixture of 0.500 mole of carbon Q55: A mixture of 0.600 mol of Q56: The equilibrium constant Kc for the Q65: Hydrogen iodide, HI, is formed in an![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents