Multiple Choice
10.0 mL of a 0.100 mol L¯1 solution of a metal ion M2+ is mixed with 10.0 mL of a 0.100 mol l¯1 solution of a substance L. The following equilibrium is established: 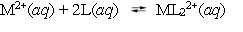 At equilibrium, the concentration of L is found to be 0.0100 mol L¯1. What is the equilibrium concentration of ML22+, in mol L¯1?
At equilibrium, the concentration of L is found to be 0.0100 mol L¯1. What is the equilibrium concentration of ML22+, in mol L¯1?
A) 0.100 mol L¯1
B) 0.050 mol L¯1
C) 0.025 mol L¯1
D) 0.0200 mol L¯1
E) 0.0100 mol L¯1
Correct Answer:
Verified
Related Questions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents