Multiple Choice
The equilibrium constant Kc for the reaction 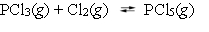 is 49 at 230 C. If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230 C, what is the concentration of PCl3 when equilibrium has been established?
is 49 at 230 C. If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230 C, what is the concentration of PCl3 when equilibrium has been established?
A) 0.049 M
B) 0.11 M
C) 0.30 M
D) 0.59 M
E) 0.83 M
Correct Answer:
Verified
Related Questions
Q39: The equilibrium constant, Kp, for the
Q40: Nitrogen dioxide decomposes according to the
Q41: Consider the reversible reaction: Q42: Nitric oxide and bromine were allowed to Q43: The equilibrium constant, Kp, for the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents