The equilibrium constant, Kp, for the reaction 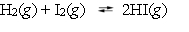 is 55.2 at 425 C. A rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. Is the system at equilibrium?
is 55.2 at 425 C. A rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. Is the system at equilibrium?
A) Yes.
B) No, the forward reaction must proceed to establish equilibrium.
C) No, the reverse reaction must proceed to establish equilibrium.
D) Need to know the volume of the container before deciding.
E) Need to know the starting concentrations of all substances before deciding.
Correct Answer:
Verified
Q34: Consider the reactions of cadmium with the
Q35: Consider the following two equilibria and their
Q36: The equilibrium constant, Kc, for the decomposition
Q37: Hydrogen sulfide will react with water as
Q38: About half of the sodium carbonate produced
Q40: Nitrogen dioxide decomposes according to the
Q41: Consider the reversible reaction: Q42: Nitric oxide and bromine were allowed to Q43: The equilibrium constant, Kp, for the Q44: The equilibrium constant Kc for the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents