Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 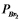 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 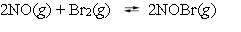
A) 7.45 * 10¯3
B) 0.109
C) 9.18
D) 91.8
E) 134
Correct Answer:
Verified
Q37: Hydrogen sulfide will react with water as
Q38: About half of the sodium carbonate produced
Q39: The equilibrium constant, Kp, for the
Q40: Nitrogen dioxide decomposes according to the
Q41: Consider the reversible reaction: Q43: The equilibrium constant, Kp, for the Q44: The equilibrium constant Kc for the Q46: The reaction system Q63: SO2 reacts with O2 to produce SO3. Q65: Hydrogen iodide, HI, is formed in an![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents