At a high temperature, the following reaction has an equilibrium constant of 1.0 *102. 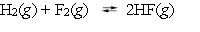
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: There is a direct correlation between the
Q3: If all of the coefficients in the
Q5: Increasing the initial amount of the limiting
Q8: For some gas-phase reactions, Kp = Kc.
Q11: If Q > K, more products need
Q13: When a reaction system reaches equilibrium, the
Q14: For a gas-phase equilibrium, a change in
Q87: Consider the equilibrium: Q90: When 0.152 mol of solid PH3BCl3 is Q93: Ammonia is synthesized in the Haber process:
A(s) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents