When 0.152 mol of solid PH3BCl3 is introduced into a 3.0 L container at a certain temperature, 8.44 *10¯3 mol of PH3 is present at equilibrium:
PH3BCl3(s)  PH3(g) + BCl3(g)
PH3(g) + BCl3(g)
Construct a reaction table for the process, and use it to calculate Kc at this temperature. 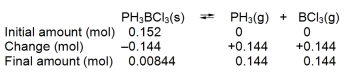
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: There is a direct correlation between the
Q5: Increasing the initial amount of the limiting
Q7: Although a system may be at equilibrium,
Q8: For some gas-phase reactions, Kp = Kc.
Q11: If Q > K, more products need
Q13: When a reaction system reaches equilibrium, the
Q14: For a gas-phase equilibrium, a change in
Q87: Consider the equilibrium: Q91: At a high temperature, the following reaction Q93: Ammonia is synthesized in the Haber process:
A(s) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents