The Compound RX3 Decomposes According to the Equation 3RX3 R + R2X3 + 3X2
In an Experiment the Following
The compound RX3 decomposes according to the equation 3RX3 R + R2X3 + 3X2
In an experiment the following data were collected for the decomposition at 100 C. What is the average rate of reaction over the entire experiment? 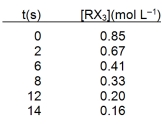
A) 0.011 mol L¯1s¯1
B) 0.019 mol L¯1s¯1
C) 0.044 mol L¯1s¯1
D) 0.049 mol L¯1s¯1
E) 0.069 mol L¯1s¯1
Correct Answer:
Verified
Q5: Ammonium cyanate (NH4CNO) reacts to form
Q6: Consider this reaction:
2NH3(g)
Q7: Consider the general reaction:
5Br¯(aq) +
Q8: When the reaction A
Q11: Sulfur trioxide can undergo decomposition according
Q12: Sulfuryl chloride, SO2Cl2(g), decomposes at high temperature
Q13: Which one of the following sets of
Q14: Based on the initial rate data
Q15: The rate constant for a reaction is
Q19: When the reaction A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents