Based on the initial rate data below, what is the value of the rate constant? 2NOBr(g) 2NO(g) + Br2(g) 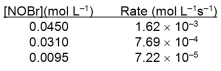
A) 0.0360 L mol¯1s¯1
B) 0.800 L mol¯1s¯1
C) 1.25 L mol¯1s¯1
D) 27.8 L mol¯1s¯1
E) 0.0360 s¯1
Correct Answer:
Verified
Q8: When the reaction A
Q10: The compound RX3 decomposes according to
Q11: Sulfur trioxide can undergo decomposition according
Q12: Sulfuryl chloride, SO2Cl2(g), decomposes at high temperature
Q13: Which one of the following sets of
Q14: Sucrose decomposes to fructose and glucose in
Q17: Consider this reaction: 8A(g) + 5B(g)
Q18: A reaction has the following rate law:
Q19: When the reaction A
Q19: For the reaction 3A(g) + 2B(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents