The complete combustion of carbon compounds produces carbon dioxide.The reaction of coal with water at high temperatures produces synthesis gas, which is a mixture of carbon monoxide and hydrogen.Use the bond energies in the table below to estimate how much more energy is released by reaction I than by reaction II.
I) C(s) +O2(g) CO2(g)
II) C(s) + H2O(g) CO2(g) +H2(g) 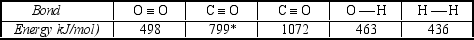 * C = O bond in CO2
* C = O bond in CO2
Correct Answer:
Verified
Q125: Identify which of the following molecules has
Q152: Describe the relationship between bond order, bond
Q164: Two reasonable Lewis structures for sulfurous acid
Q165: Ethanethiol and dimethyl sulfide share the same
Q167: Draw the Lewis structure for the nitrate
Q168: Using the positions of the atoms in
Q170: Draw the Lewis structure for the carbonate
Q171: Which of the following bonds is considered
Q172: Chlorofluorocarbons (CFCs or freons) are linked to
Q173: Based on electronegativities, which of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents