Two reasonable Lewis structures for sulfurous acid (H2SO3) are given below.Add the formal charges to each atom. 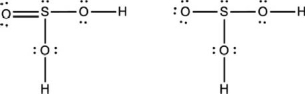
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q125: Identify which of the following molecules has
Q127: The evaporation of seawater gives a mixture
Q159: What are the names for the following
Q160: Which molecule has a stretching vibration that
Q162: Carbonyl fluoride (COF2) is similar in structure
Q163: Draw the Lewis structure of boron trifluoride.
Q165: Ethanethiol and dimethyl sulfide share the same
Q167: Draw the Lewis structure for the nitrate
Q168: Using the positions of the atoms in
Q169: The complete combustion of carbon compounds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents