The amide functional group is the fundamental linking unit in proteins.Two partial Lewis structures for formamide are shown below.These structures do not show the lone pair electrons or the formal charges.Experiment shows that formamide is planar, so which is the better representation of the electronic structure, I or II? Is this conclusion consistent with the formal charges on the atoms? Explain the rationale for your answers. 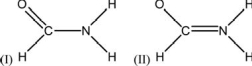
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q131: Identify the hybridization of atomic orbitals for
Q132: A Lewis structure of aspirin without the
Q133: Give the molecular orbital valence electron configuration
Q134: Use the 1s orbital of hydrogen
Q135: Draw the Lewis structure of BrF5.Give the
Q136: Answer the following questions about the
Q137: In 1995 a Japanese cult attacked the
Q139: A Lewis structure of aspirin without the
Q140: ICl3 and SO3 have one central atom.Do
Q141: Three 2pz orbitals on three oxygen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents