Give the formula for the precipitate that will form when the following aqueous solutions are mixed.If no precipitate forms, write "none." 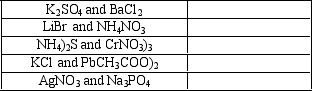
Correct Answer:
Verified
Q121: Define the terms oxidizing agent and reducing
Q143: Some microorganisms living under anaerobic conditions extract
Q170: Write the balanced molecular equation and the
Q171: Write the balanced molecular equation and the
Q173: Aqueous potassium phosphate is added to 545
Q175: Ammonia, which is highly toxic to
Q176: Outline a method that could be used
Q177: A saturated sucrose (C12H22O11, 342.30 g/mol)
Q178: A food chemist determined the concentration of
Q179: Aqueous sodium phosphate is added to 25.00
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents