Given the following reactions, what is the overall enthalpy change for the following reaction?
C4 H4 (g) + 2H2 (g) C4 H8 (g) 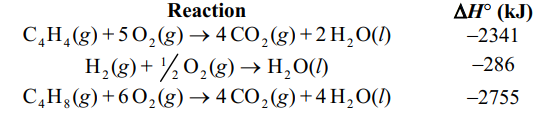
A) ( - 128 kJ)
B) ( - 5672 kJ)
C) ( - 986 kJ)
D) ( - 158 kJ)
E) ( - 444 kJ)
Correct Answer:
Verified
Q102: Determine the standard enthalpy of formation
Q103: A food sample was burned in
Q104: Use the following information to determine the
Q105: Which of the following shows the
Q106: Given the following reactions, what is
Q108: The standard enthalpy of combustion of liquid
Q109: Indicate which of the following is
Q110: Determine the change in enthalpy for
Q111: Given the standard enthalpies of formation for
Q112: Isooctane is a good model for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents