Henry's law constant for oxygen dissolving in blood is 3.74 *10-2 mol/L . atm at body temperature, 37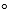 C.Calculate the molar concentration of oxygen in blood for a free diver where the air pressure is 3.0 atm.The mole fraction of oxygen in air is 0.209.
C.Calculate the molar concentration of oxygen in blood for a free diver where the air pressure is 3.0 atm.The mole fraction of oxygen in air is 0.209.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q120: Describe how you would use the data
Q121: What is the molality of a
Q124: Carbonated beverages are manufactured to dissolve
Q125: The vapor pressure of acetone (C3H6O,
Q126: What is the boiling point of
Q126: Seawater can be characterized by the following
Q127: Water at 25.0
Q128: Henry's law constant for carbon dioxide
Q130: Which has the higher vapor pressure at
Q130: How does the presence of solutes raise
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents