What is the overall standard free energy change when the following two reactions are coupled?
A + B C G 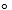 rxn = G1
rxn = G1
C+ D E G11eed8bd_5dd2_ab4a_a0ba_097b88c7a998_TB6562_11 rxn - G2
A) ( G1 + G2)
B) ( G1+ G2) .
C) ( G2 - G1)
D) ( G1 G2)
E) ( G1/ G2)
Correct Answer:
Verified
Q102: Only one substance has a standard entropy
Q104: Considering the tabulated values for the thermodynamic
Q106: Hydrogen bromide can theoretically be made
Q108: When one mole of solid ammonia
Q109: The standard molar enthalpy and entropy
Q111: The standard molar enthalpy and entropy
Q112: Carbon atoms can be found in a
Q158: Give an example of a spontaneous and
Q179: Name and state the first three laws
Q181: The standard entropy of diamond is 2.4
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents