Hydrogen bromide can theoretically be made by the reaction of hydrogen gas and bromine by the reaction H2 (g) + Br2(l) 2 HBr(g).What is the standard entropy change?
S 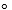 H2, (g) =130.6 J/mol.K
H2, (g) =130.6 J/mol.K
S 11eed8be_a0d0_374d_a0ba_3dcd3735157c_TB6562_11 Br2, (l) =152.2 J/mol . K
S 11eed8be_a0d0_374d_a0ba_3dcd3735157c_TB6562_11 HBr, (g) =198.7 J/mol . K
Correct Answer:
Verified
Q102: Only one substance has a standard entropy
Q104: Considering the tabulated values for the thermodynamic
Q107: What is the overall standard free
Q108: When one mole of solid ammonia
Q109: The standard molar enthalpy and entropy
Q111: The standard molar enthalpy and entropy
Q158: Give an example of a spontaneous and
Q167: Draw a graph of entropy versus temperature
Q179: Name and state the first three laws
Q181: The standard entropy of diamond is 2.4
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents