Approximately how many times faster or slower is the average reaction rate during the first four seconds (t =0 to t = 4) than the second four seconds t = 4 to t = 8) for the following reaction? 2 NOBr(g) Br2 (g) + 2 NO(g) 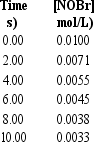
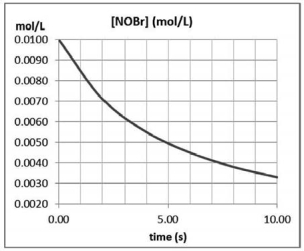
A) about 1.4 times faster
B) about 1.4 times slower
C) about 2.64 times slower
D) about 2.6 times faster
E) The average rate is constant.
Correct Answer:
Verified
Q28: The reaction CHCl3(g) +CL2(g)
Q29: For the reaction 3A+ 5B
Q30: The rate of disappearance of HI
Q31: The rate of a reaction is found
Q32: Which statement below is true about
Q34: Given the following data, determine the
Q35: The reaction 2 NO(g) +O2(g)
Q37: The rate of disappearance of HI
Q38: Given the following data, determine the
Q42: The rate of a reaction is found
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents