The rate of disappearance of HI in the reaction 2 HI(g) I2(g) +H2(g) is shown in the following figure.What is the approximate average rate of reaction over the first 15.0 seconds of the reaction? 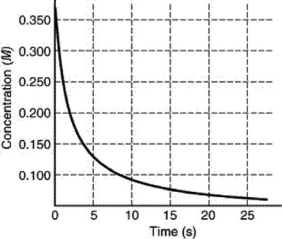
A) 0.275 M/s
B) 0.138 M/s
C) 0.0117 M/s
D) 0.00917 M/s
E) 0.0183 M/s
Correct Answer:
Verified
Q32: Which statement below is true about
Q33: Approximately how many times faster or
Q34: Given the following data, determine the
Q35: The reaction 2 NO(g) +O2(g)
Q38: Given the following data, determine the
Q39: In a rate law, the partial orders
Q40: Given the following data, determine the
Q41: The reaction C2 H5 Cl(g)
Q42: The rate of a reaction is found
Q42: The second-order reaction A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents