Given the following data, determine the order of the reaction with respect to NO(g) . 2 NO(g) +CL2(g) 2 NOCL2(g) 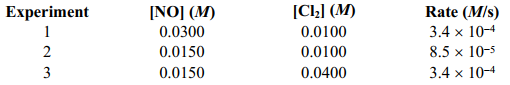
A) first
B) second
C) third
D) fourth
E) zero
Correct Answer:
Verified
Q35: The reaction 2 NO(g) +O2(g)
Q37: The rate of disappearance of HI
Q38: Given the following data, determine the
Q39: In a rate law, the partial orders
Q41: The reaction C2 H5 Cl(g)
Q42: The rate of a reaction is found
Q42: The second-order reaction A
Q43: Determine the overall order of the
Q44: The rate of disappearance of HI
Q45: The half-life (t1/2) of a first-order reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents