Multiple Choice
Given the following two measurements of the equilibrium constant for a reaction, calculate H for the reaction. 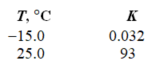
A) 127 kJ/mol
B) (-0.621 kJ/mol)
C) 16.0 kJ/mol
D) (-127 kJ/mol)
E) (-0.621 kJ/mol) .
Correct Answer:
Verified
Related Questions
Q78: For the reaction 2 H2 S(g)
Q79: In the equation relating equilibrium to
Q80: What is the value of the
Q81: A student can accept that
Q82: The
Q84: A chemist is planning to study
Q85: As the temperature of an endothermic
Q86: A plot of ln K vs.1/T
Q87: A sketch of the free energy for
Q88: The values of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents