Use the table of standard reduction potentials below to identify which statement below is FALSE. 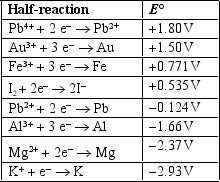
A) Pb4+will oxidize all of the species on the right side of the arrows in the other half-reactions.
B) K will reduce all of the species on the left side of the arrows in the other half-reactions.
C) I2 is a better reducing agent than PB2+.
D) Al is easier to oxidize than Fe.
E) Mg is a better reducing agent than Pb.
Correct Answer:
Verified
Q26: Using the following data, determine the
Q27: Which statement below regarding chemical energy and
Q28: Using the following data, determine the standard
Q29: Use the table of standard reduction potentials
Q30: What is the standard cell potential for
Q32: The bromate ion, BrO3-, can form
Q33: Silver tarnish Ag2S) can be removed by
Q34: The magnitude of the charge on a
Q35: Silver tarnish( Ag2S) can be removed by
Q36: In one episode of the 1960s television
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents