Using the following data, determine the standard cell potential Eo cell for the electrochemical cell constructed based on the following unbalanced reaction expression: 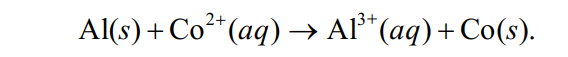

A) (-1.385 V)
B) (+1.385 V)
C) (+1.939 V)
D) (-2.770 V)
E) (+2.770 V)
Correct Answer:
Verified
Q23: Which statement below regarding reduction potentials is
Q24: Which one of the following items does
Q26: Using the following data, determine the
Q27: Which statement below regarding chemical energy and
Q29: Use the table of standard reduction potentials
Q30: What is the standard cell potential for
Q31: Use the table of standard reduction potentials
Q32: The bromate ion, BrO3-, can form
Q33: Silver tarnish Ag2S) can be removed by
Q66: The work involved in moving exactly 1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents