The standard cell potential for the nickel-cadmium battery is 1.35 V, and the cell reaction can be written as 2 NiO(OH) (s) +2 H2 O(l) + Cd(s) 2 Ni(OH) 2 (s) + Cd(OH) 2 (s)
Which statement below is true based on the Nernst equation? Note: Q = reaction quotient, and
K = equilibrium constant for the cell reaction.
A) As the battery is used, the cell voltage approaches zero because Q approaches K in value.
B) When the battery no longer works, the cell voltage is zero because Q = K.
C) As the battery is used, the cell voltage does not change because Q equals 1.
D) When the battery is fully charged, Q 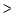 K.
K.
E) When the battery is fully charged, Q 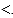 K.
K.
Correct Answer:
Verified
Q28: The electrodes on batteries are labeled +
Q38: The electrodes on batteries are labeled +
Q65: A concentration cell with a cell potential
Q67: Electrochemical cell potentials can be used
Q68: A pH meter uses an electrode arrangement
Q69: A concentration cell with a cell potential
Q73: Which one of the following is NOT
Q74: The spontaneous redox reaction in a
Q75: The unit of current, the ampere (A),
Q104: The Energizer Bunny in the television commercial
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents