The spontaneous redox reaction in a voltaic cell has
A) Eo cell 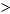 0, G° 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 0, and K 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 1
0, G° 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 0, and K 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 1
B) Eocell 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 0, G° 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 0, and K 11eed928_5622_4673_a0ba_f5daa2fc20ab_TB6562_11 1
C) Eo cell 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 0, G° 11eed928_5622_4673_a0ba_f5daa2fc20ab_TB6562_11 0, and K 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 1
D) Eo cell 11eed927_f2e6_11e2_a0ba_15d493ae4d84_TB6562_11 0, (\Delta\) G° 11eed927_f2e6_11e2_a0ba_15d493ae4d84_TB6562_11 0, and K 11eed927_f2e6_11e2_a0ba_15d493ae4d84_TB6562_11 1
E) Eocell 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 0, G° = 0, and K 11eed928_8239_0c84_a0ba_7181550d35e5_TB6562_11 1
Correct Answer:
Verified
Q28: The electrodes on batteries are labeled +
Q69: A concentration cell with a cell potential
Q70: The standard cell potential for the
Q73: Which one of the following is NOT
Q75: The unit of current, the ampere (A),
Q76: When a voltaic cell reaches equilibrium,
A)Eo cell
Q78: Suppose the redox reaction in a
Q104: The Energizer Bunny in the television commercial
Q107: The charge supplied by a battery can
Q115: A NiMH battery uses _ as the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents