5.00 kg of liquid water is heated to 100.0 °C in a closed system. At this temperature, the density of liquid water is 958 kg/m3. The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa. A moveable piston of negligible weight rests on the surface of the water. The water is then converted to steam by adding an additional amount of heat to the system. When all of the water is converted, the final volume of the steam is 8.50 m3. The latent heat of vaporization of water is 2.26 × 106 J/kg. 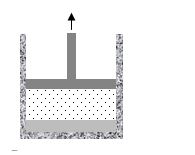
-How much heat is added to the system in the isothermal process of converting all of the water into steam?
A) 2.17 × 103 J
B) 1.70 × 104 J
C) 4.52 × 105 J
D) 3.78 × 106 J
E) 1.13 × 107 J
Correct Answer:
Verified
Q11: An ideal gas absorbs 750 J of
Q13: Enclosed beneath the moveable piston in the
Q13: Heat is added to a sample of
Q14: 5.00 kg of liquid water is heated
Q15: Complete the following statement: Walls that separate
Q17: A thermally isolated sample of an ideal
Q19: Complete the following statement: The first law
Q19: 5.00 kg of liquid water is heated
Q20: Which one of the following situations is
Q21: An ideal monatomic gas expands isobarically from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents