Enclosed beneath the moveable piston in the drawing is 4.8 moles of a monatomic ideal gas. The gas performs work on the piston as 2300 J of heat are added from the surroundings. During the process, the temperature of the gas decreases by 45 K. How much work does the gas perform? 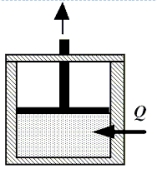
A) 5.0 × 103 J
B) 3.2 × 103 J
C) 1.4 × 103 J
D) 6.0 × 102 J
E) 4.4 × 103 J
Correct Answer:
Verified
Q5: Neon is a monatomic gas with a
Q8: A container is divided into two chambers
Q11: An ideal gas absorbs 750 J of
Q13: Heat is added to a sample of
Q14: 5.00 kg of liquid water is heated
Q15: Complete the following statement: Walls that separate
Q16: An isobaric process is represented on a
Q16: 5.00 kg of liquid water is heated
Q17: A thermally isolated sample of an ideal
Q19: Complete the following statement: The first law
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents