A container is divided into two chambers that are separated by a valve. The left chamber contains one mole of a monatomic ideal gas. The right chamber is evacuated. At some instant, the valve is opened and the gas rushes freely into the right chamber. Which one of the following statements concerning this process is true? 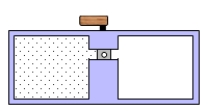
A) Work is done by the gas.
B) The temperature of the gas decreases.
C) The change in the entropy of the gas is zero.
D) The walls of the containing vessel must get colder.
E) The change in the internal energy of the gas is zero.
Correct Answer:
Verified
Q4: What are the SI units of the
Q5: When the gas enclosed beneath the piston
Q5: Neon is a monatomic gas with a
Q7: A system containing an ideal gas at
Q11: An ideal gas absorbs 750 J of
Q13: Heat is added to a sample of
Q13: Enclosed beneath the moveable piston in the
Q16: An isobaric process is represented on a
Q17: Rick spends four hours researching on the
Q18: A match ignites within in an oxygen-filled
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents