Radium-226 decays into radon-222 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are: 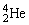 , 4.002603 u;
, 4.002603 u; 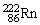 , 222.017570 u;
, 222.017570 u; 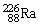 , 226.025402 u.
, 226.025402 u.
A) 4.24 MeV
B) 3.76 MeV
C) 4.87 MeV
D) 5.05 MeV
E) 5.39 MeV
Correct Answer:
Verified
Q39: Francium-223 decays to radium-223 by emitting what
Q44: A radioactive sample has a half-life of
Q60: What is the density of the nucleus
Q61: An atom has 98 protons and 249
Q62: A stationary plutonium-239 nucleus decays into a
Q67: 241Am decays by alpha particle emission. The
Q69: Plutonium-239 decays into uranium-235 plus an alpha
Q70: A carbon-14 nucleus decays to a nitrogen-14
Q73: Americium-243 has a decay constant of 9.39
Q80: Rutherfordium-261 has a half-life of 1.08 min.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents