Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 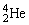 , 4.002603 u;
, 4.002603 u; 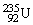 , 235.043924 u; what is the mass value of
, 235.043924 u; what is the mass value of 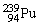 ? 1 u = 931.494 MeV/c2.
? 1 u = 931.494 MeV/c2.
A) 239.052157 u
B) 239.027750 u
C) 239.001889 u
D) 238.9991889 u
E) 238.988842 u
Correct Answer:
Verified
Q44: A radioactive sample has a half-life of
Q65: Radium-226 decays into radon-222 plus an alpha
Q66: A certain substance has a half-life of
Q67: 241Am decays by alpha particle emission. The
Q70: A carbon-14 nucleus decays to a nitrogen-14
Q71: Uranium-238 decays into thorium-234 plus an alpha
Q72: An element with atomic number 6 undergoes
Q72: The decay constant of radon is 0.181
Q73: Americium-243 has a decay constant of 9.39
Q80: Rutherfordium-261 has a half-life of 1.08 min.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents