What type of signal(s) would you observe in the mass and (or) infrared spectrum of the following compound?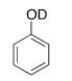
A) A signal at 95 amu
B) A signal at 94 amu
C) Two signals at 95 and 94 amu
D) A signal at 3600-3200 cm-1
Correct Answer:
Verified
Q25: In a typical mass spectrum, a
Q26: An IR spectrum has the following
Q27: Examine the IR below and classify the
Q28: When the phenol shown below is
Q29: Examine the IR below and classify the
Q30: Which of the following π bonds is
Q31: Examine the IR below and classify the
Q32: Which of the following compounds will have
Q33: Examine the IR below and classify the
Q34: What would be the molecular formula
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents