An unknown molecular compound has the following Lewis structure.Which of the following elements could be the identity of X? 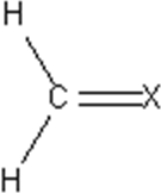
A) C
B) N
C) O
D) Cl
E) Ne
Correct Answer:
Verified
Q45: Which of the following statements regarding ionic
Q55: Draw the Lewis symbol for MgCl2.
A)Cl-Mg-Cl
B)
Q56: The formula for calcium sulfide is:
A)Ca2S
B)CaS2
C)CaS
D)Ca2S2
Q57: The formula for calcium nitride is:
A)CaN
B)Ca2N
C)CaN2
D)Ca3N2
Q58: Which of the following statements regarding ionic
Q60: An unknown molecular compound has the following
Q61: Draw the Lewis structure for the NO2-
Q62: Which of these molecules or ions has
Q63: Which of the following molecules would exhibit
Q64: Which of the following contains a triple
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents