Consider the reaction N2(g) + 2O2(g) 2NO2(g) .The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 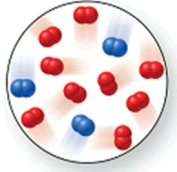
A) N2(g) , 6 O2(g)
B) O2(g) , 1 N2(g)
C) N2(g) , 3 O2(g)
D) O2(g) , 2 N2(g)
E) N2(g) , 7 O2(g)
Correct Answer:
Verified
Q72: Consider the following reaction: 3NO2(g) +
Q73: Iron metal reacts with hydrochloric acid
Q73: If the theoretical yield for a reaction
Q74: Iron metal reacts with hydrochloric acid
Q75: Iron metal reacts with chlorine gas
Q76: What mass (in grams) of SF6
Q78: Aluminum reacts with oxygen according to
Q80: In the process of obtaining lead
Q96: Which of the following is an exothermic
Q99: A carton of low-fat yogurt says it
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents