Consider the following specific heats of metals. 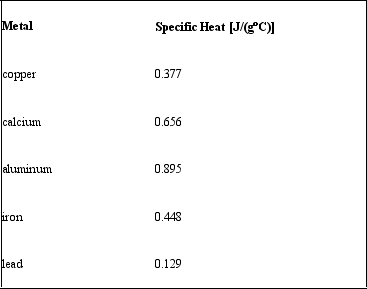 If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
A) copper
B) calcium
C) aluminum
D) iron
E) lead
Correct Answer:
Verified
Q83: When sulfur dioxide is formed from its
Q87: Which of the following processes is exothermic?
A)boiling
Q88: Consider the following specific heats of metals.
Q89: If 75.0 J of heat energy is
Q90: How much heat energy would be
Q92: When carbon dioxide is formed from its
Q95: An energy input of 227 kJ is
Q97: Which of the following is an exothermic
Q97: The following reaction releases 2800 kJ
Q100: Which of the following processes is endothermic?
A)burning
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents