If 75.0 J of heat energy is added to 25.0 g samples of different metals.Given their specific heat values, rank the metals in order from least to greatest final temperature. 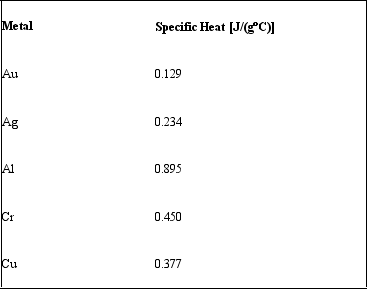
A) Au < Ag < Cu < Cr < Al
B) Al < Cr < Cu < Ag < Au
C) Au < Ag < Al < Cr < Cu
D) Cr < Cu < Al < Ag < Au
E) none of these-all final temperatures would be equal
Correct Answer:
Verified
Q83: When sulfur dioxide is formed from its
Q85: An equal quantity of heat is transferred
Q85: A 43 g serving of a chocolate
Q86: The following reaction absorbs 393 kJ
Q88: Consider the following specific heats of metals.
Q90: How much heat energy would be
Q92: When carbon dioxide is formed from its
Q93: Consider the following specific heats of metals.
Q97: Which of the following is an exothermic
Q98: A can of soda has 1.50 x
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents