What is the formal charge on the oxygen atom in each of the following Lewis structures? 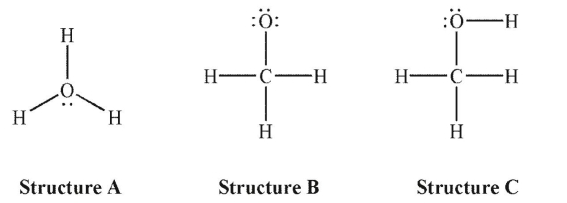
A) A: 0, B: 1-, C: 1+
B) A: 1+, B: 1-, C: 0
C) A: 1-, B: 1+, C: 0
D) A: 1-, B: 1-, C: 1-
E) A: 1+, B: 1+, C: 1-
Correct Answer:
Verified
Q14: In which of the following structures does
Q15: Which of the following arrow conventions is
Q16: Which of the following statements is true?
A)Ionization
Q17: d-orbitals have two nodal planes. How many
Q18: The rule or principle that states that
Q20: Which of these sets of quantum numbers
Q21: Which of the following statements about the
Q22: Which of the following pairs are related
Q23: How many antibonding molecular orbitals are generated
Q24: Which two of the following structures are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents