Why is the carbon atom typically the nucleophilic site of an enolate anion? 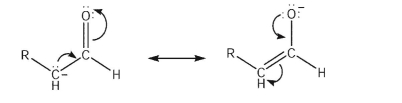
A) Most of the negative charge resides on the carbon atom.
B) The resonance form with the negative charge on carbon is the major contributor.
C) The alkoxy anion will not act as a Lewis base, due to oxygen electronegativity.
D) The HOMO of the enolate has its largest lobe at the beta-carbon.
E) The bonding MO of the enolate has its highest electron density around the oxygen.
Correct Answer:
Verified
Q1: Which of the following could not serve
Q2: Which of the following compounds is the
Q3: Which of the following is the correct
Q4: Which of the following compounds could not
Q5: Rank the circled protons in the following
Q7: Which of the following is the correct
Q8: Why should lithium diisopropylamide (LDA) be used
Q9: What is the final product of the
Q10: Which of the following structures will have
Q11: Which of the following is the correct
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents