Calculate ΔG° for the reaction SiCl4(g) + 2Mg(s) → 2MgCl2(s) + Si(s) 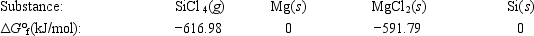
A) 566.60 kJ
B) 50.38 kJ
C) 25.19 kJ
D) −25.19 kJ
E) −566.60 kJ
Correct Answer:
Verified
Q73: Calculate ΔG° for the reaction of ammonia
Q74: Elemental boron can be formed by reaction
Q75: Calculate ΔG° for the combustion of propane.
Q76: Nitric oxide reacts with chlorine to form
Q77: Consider the figure that shows ΔG° for
Q79: Consider the figure that shows ΔG° for
Q80: A reaction is proceeding toward equilibrium. At
Q81: Select the correct statement of a law
Q82: Consider the reaction CuI(s) ⇄ Cu+(aq) +
Q83: The formation constant for the reaction Ag+(aq)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents