Consider the reaction shown in the figure. If the initial conditions are the same in both flasks, with the exception of the concentration of the acetic acid, explain why there is a difference in the size of the balloons after the reaction has progressed for 10 seconds. 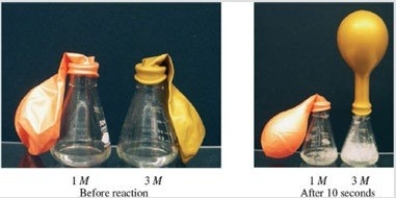 ©Jim Birk
©Jim Birk
Correct Answer:
Verified
Q100: An activated complex is a short-lived, high-energy
Q101: The value of the equilibrium constant for
Q102: Consider the reaction and its equilibrium constant:
Q103: Is the system shown in the figure
Q104: Using collision theory, explain why reaction rates
Q105: If the initial concentrations of reactants and
Q106: If the equilibrium constant at 25°C is
Q107: What is the relationship between the following
Q108: When yeast bread dough is placed in
Q110: Why are the concentrations of pure liquids
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents