Assuming that all of the gas in the tank in the figure is used to fill balloons with an average volume of 2.0 L of helium, approximately how many balloons could be filled at STP? 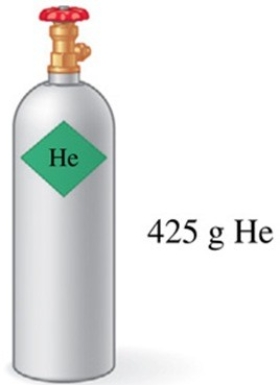
A) about 45 balloons
B) about 106 balloons
C) about 210 balloons
D) about 850 balloons
E) about 1200 balloons
Correct Answer:
Verified
Q36: A sample of gas initially occupies 3.35
Q37: For which of the following changes is
Q38: If the volume of a sample of
Q39: Given a fixed amount of gas in
Q40: The pressure-volume relationship expressed by Boyle's law
Q42: A sample of gas initially occupies 2.50
Q43: Calculate the number of moles in 75.0
Q44: Calculate the number of moles and the
Q45: A sample of gas initially occupies 2.50
Q46: The two balloons shown in the figure
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents