Which of the following is a balanced equation with lowest whole number coefficients that represents the reaction shown in the figure? 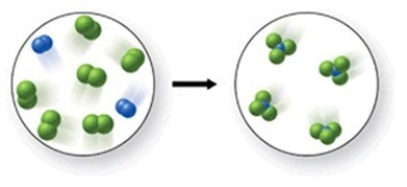
A) 2N2 + 6Cl2 → 3NCl4
B) N2 + 3Cl2 → 2NCl3
C) 2N2 + 6Cl2 → 4NCl3
D) N2 + 6Cl2 → 4NCl3
E) 2N2 + 5Cl2 → 3NCl3
Correct Answer:
Verified
Q17: The figure shows a reaction between xenon
Q18: The figure shows a reaction between hydrogen
Q19: Consider the following chemical equations. Select the
Q20: Sulfur dioxide gas reacts with oxygen gas
Q21: The gases carbon dioxide and hydrogen can
Q23: Balance the following skeletal equation: Ba(NO3)2(aq)+ K2SO4(aq)→
Q24: Balance the following skeletal equation: NH3(g)+ O2(g)→
Q25: After the following equation is properly balanced,
Q26: When aqueous solutions of hydrochloric acid and
Q27: Balance the following skeletal equation: Li(s)+ H2O(l)→
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents