What is the formula of the molecule in the figure (b)below? Indicate the values of the subscripts in the generic formula SxCly. 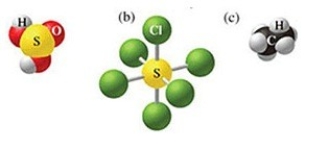
Correct Answer:
Verified
Q125: How many oxygen atoms are in 0.25
Q126: Calculate the number of moles provided by
Q127: What is the mass of 1.90 ×
Q128: How many sulfur atoms are in 0.25
Q129: To obtain 0.50 moles of potassium nitrate
Q131: Elemental analysis of nicotine, which is found
Q132: What is the formula of the molecule
Q133: Calculate the molar mass of SO2 in
Q134: Calculate the molar mass of Na in
Q135: How many moles atoms of iron are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents